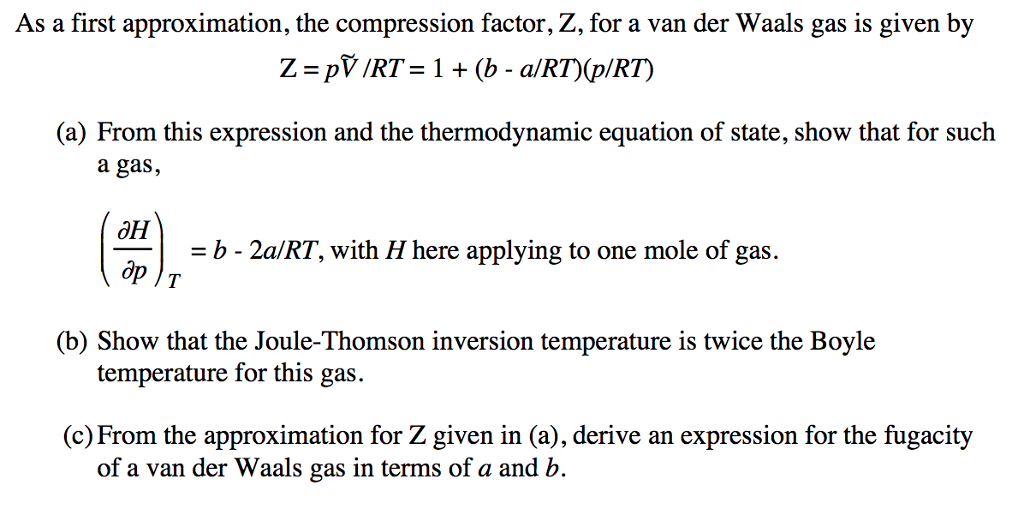
Solved As a first approximation, the compression factor, Z
4.9 (422) In stock

Compressibility Factor of Gas, Overview, Equation & Chart - Lesson
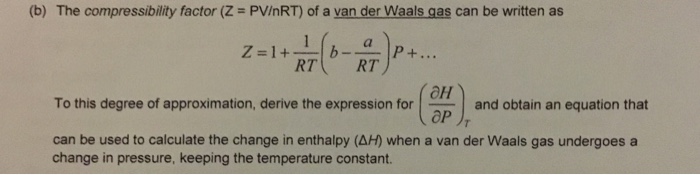
Solved (b) The compressibility factor (Z - PV/nRT) of a van

Compressibility Factor Z
Using the PA=LU factorization to solve linear systems of equations for many right-hand sides efficiently

Solved) - For values of z near 1, it is a good approximation to write z(P) = - (1 Answer)
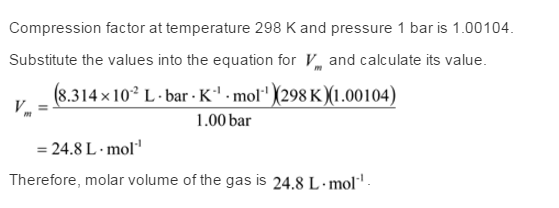
Solved) - For values of z near 1, it is a good approximation to write z(P) = - (1 Answer)

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics

Compressibility factor - Wikipedia
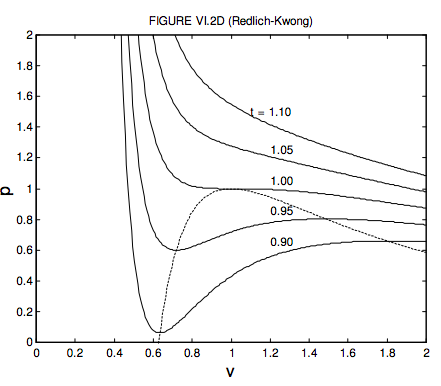
6.3: Van der Waals and Other Gases - Physics LibreTexts

Compressibility factors of air using improved virial equation and P-R

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics

Methane compressibility factor evolution

Compressibility Factor Z

SOLVED: The Dieterici equation is another two-parameter equation of state for a one-component gas (much like the van der Waals equation): PV = RT exp(-a/Vm) Question: Write the Dieterici equation for the
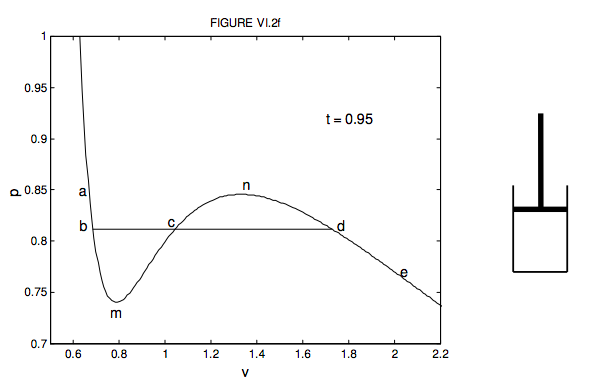
6.3: Van der Waals and Other Gases - Physics LibreTexts
What is the compression ratio, and how is it calculated? - Quora
the compression factor one mole of a vander waals gas 0 C and 100 atm pressure is found to be 0.5
Solved) - For values of z near 1, it is a good approximation to write z(P) = - (1 Answer)
COMPRESSION AND EXPANSION OF GASES – Chemical Engineering Projects
 Colombian Fajas Gaine Amincissante Femme Womens Tummy Control
Colombian Fajas Gaine Amincissante Femme Womens Tummy Control Brach's Milk Maid Caramels, Individually Wrapped Candy, 10oz Bag
Brach's Milk Maid Caramels, Individually Wrapped Candy, 10oz Bag Gymshark ADAPT OMBRE SEAMLESS Padded SPORTS BRA Small Rose Pink
Gymshark ADAPT OMBRE SEAMLESS Padded SPORTS BRA Small Rose Pink The 7” One Short (Lined) - Hydrow Apparel Store
The 7” One Short (Lined) - Hydrow Apparel Store Vintage Cream Bolce Chunky Textured Knit Jacket Sweater With
Vintage Cream Bolce Chunky Textured Knit Jacket Sweater With ZIVAME Women Maternity/Nursing Non Padded Bra - Buy ZIVAME Women
ZIVAME Women Maternity/Nursing Non Padded Bra - Buy ZIVAME Women