Health-related quality of life and quality-adjusted progression
4.8 (193) In stock
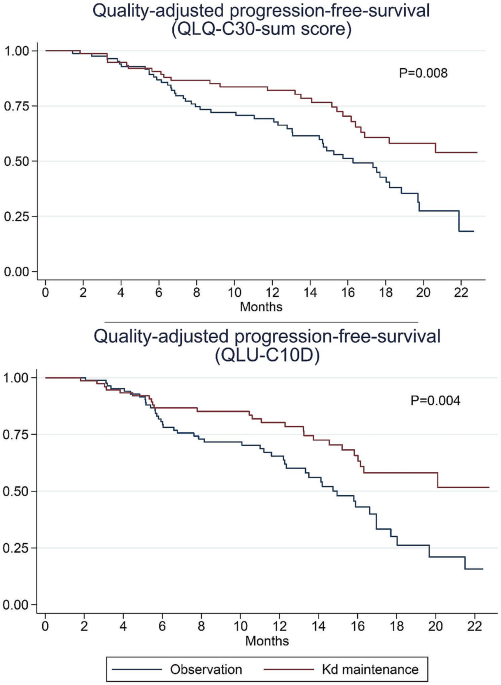
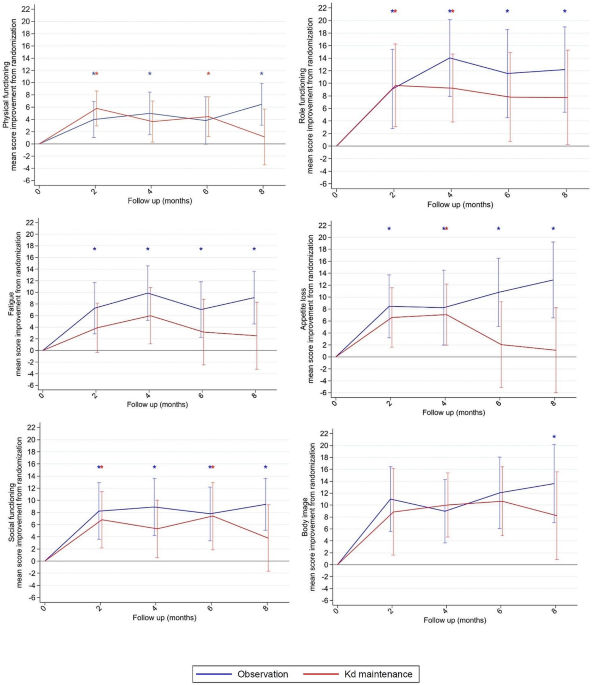
Background Decisions regarding maintenance therapy in patients with multiple myeloma should be based on both treatment efficacy and health-related quality of life (HRQL) consequences. In the CARFI trial, patients with first relapse of multiple myeloma underwent salvage autologous stem cell transplantation (salvage ASCT) before randomization to carfilzomib-dexamethasone maintenance therapy (Kd) or observation. The primary clinical endpoint was time to progression, which was extended by 8 months by Kd. The aim of this paper is to present the all HRQL endpoints of the CARFI trial including the HRQL effect of Kd maintenance therapy relative to observation. The primary HRQL endpoint was assessed by EORTC QLQ-C30 Summary score (QLQ-C30-sum) at 8 months follow-up. A key secondary HRQL endpoint was quality-adjusted progression-free-survival (QAPFS). Methods HRQL was assessed with EORTC QLQ-C30, EORTC QLQ-MY20 and FACT/GOG-Ntx at randomization and every second month during follow-up. HRQL data were analyzed with linear mixed effect models until 8 months follow-up. QAPFS per individual was calculated by multiplying progression-free survival (PFS) by two quality-adjustment metrics, the QLQ-C30-sum and EORTC Quality of Life Utility Measure-Core 10 dimensions (QLU-C10D). The QAPFS per treatment group was estimated with the Kaplan-Meier method. P < 0.05 was used for statistical significance, and a between-group minimal important difference of 10 points was interpreted as clinically relevant for the QLQ-C30-sum. Results 168 patients were randomized. HRQL questionnaire compliance was 93%. For the QLQ-C30-sum, the difference of 4.62 points (95% confidence interval (CI) -8.9: -0.4, p = 0.032) was not clinically relevant. PFS was 19.3 months for the Kd maintenance group and 16.8 months for the observation group; difference = 2.5 months (95% CI 0.5; 4.5). QAPFS based on the QLQ-C30-sum for the Kd maintenance group was 18.0 months (95% CI 16.4; 19.6) and for the observation group 15.0 months (95% CI 13.5; 16.5); difference = 3.0 months (95% CI 0.8–5.3). QAPFS based on the QLU-C10D for the Kd maintenance group was 17.5 months (95% CI 15.9; 19.2) and 14.0 months (95% CI 12.4; 15.5) for the observation group; difference = 3.5 months (95% CI 1.1–5.9). Conclusions Kd maintenance therapy after salvage ASCT did not adversely affect overall HRQL, but adjustment for HRQL reduced the PFS compared to unadjusted PFS. PFS of maintenance therapy should be quality-adjusted to balance the benefits and HRQL impact.
Measuring health related quality of life

Health-related quality of life in the ENDEAVOR study: carfilzomib-dexamethasone vs bortezomib-dexamethasone in relapsed/refractory multiple myeloma

OUH - Group members

Change in FACT‐BMT score between baseline and 1‐year post‐transplant.
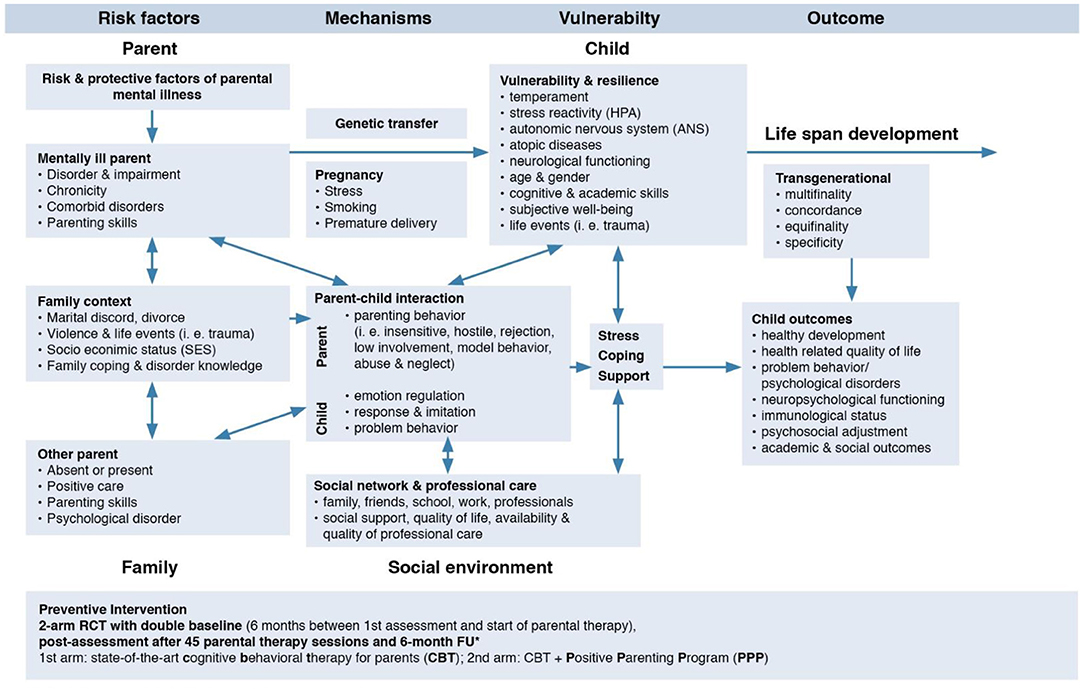
Frontiers Children of Mentally III Parents at Risk Evaluation (COMPARE): Design and Methods of a Randomized Controlled Multicenter Study—Part I

Five-and 10-year relative survival ratios with 95% confidence intervals

OUH - Group members

PDF) Health state utility values: A description of their development and application for rheumatic diseases
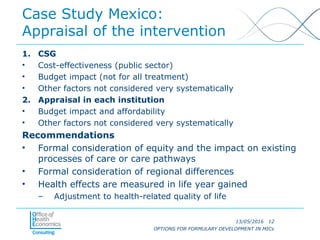
OPTIONS FOR FORMULARY DEVELOPMENT IN MIDDLE-INCOME COUNTRIES
Health-related quality of life and quality-adjusted progression free survival for carfilzomib and dexamethasone maintenance following salvage autologous stem-cell transplantation in patients with multiple myeloma: a randomized phase 2 trial by the Nordic

Extent of missing QLQ-C30 questionnaires (intention-to-treat)

The effect of comorbidity on health-related quality of life for injury patients in the first year following injury: comparison of three comorbidity adjustment approaches

d28hgpri8am2if.cloudfront.net/book_images/onix/cvr
Too High to Drive? New App Allows Marijuana Users to Test Their Impairment Level
Participatory reporting of the 2016 bleaching event in the Western Indian Ocean
 Vintage 90s Clothing Tommy Hilfiger Jeans Brand Men Size Small / Oversized Womens Retro Tommy Sport Color Block Sleeveless Basketball Jersey - Canada
Vintage 90s Clothing Tommy Hilfiger Jeans Brand Men Size Small / Oversized Womens Retro Tommy Sport Color Block Sleeveless Basketball Jersey - Canada Inhumane y2k Shooter Streetwear Tee
Inhumane y2k Shooter Streetwear Tee Cute cartoon sleeping bunny | Sticker
Cute cartoon sleeping bunny | Sticker Gymshark Vital Seamless 2.0 T-Shirt - Smokey Grey Marl
Gymshark Vital Seamless 2.0 T-Shirt - Smokey Grey Marl- Almay Healthy Hue Blush 300 Pink Flush - 0.17oz : Target
 Bolshevism: The Road to Revolution - a reading guide
Bolshevism: The Road to Revolution - a reading guide
